Fission is a process in which a nucleus with a large mass number splits into two nuclei, which have smaller mass numbers.
You need to know:
- neutrons are usually released when fission takes place
- fission of a nucleus may be spontaneous, that is, it may happen at random due to internal processes within the nucleus
- fission can also be induced by bombarding a nucleus with a neutron. Induced fission is used to generate nuclear power and for weapons
- the products formed during fission gain kinetic energy. It is this energy that is harnessed in nuclear power stations
Fusion is a process in which two nuclei combine to form a nucleus of larger mass number.
- Fusion is the main nuclear process that occurs in the Sun and other stars.
- The products of fusion reactions also gain kinetic energy that can be harnessed.
Albert Einstein in his famous equation E = mc2 showed that mass and energy are equivalent.
In this equation, E is energy in joules; is mass in kilograms; is the velocity of electromagnetic radiation in a vacuum (3 × 108 m/s).
Question
If we were to completely convert 1 kg of oil into energy, how much energy would we obtain?
AnswerE = mc2
= 1 × (3 × 108)2
= 9 × 10 16J
Of course when we burn oil we do not convert all of its mass into energy - most of the mass remains so we only get a minute fraction of this quantity of energy by burning fuel.
Binding energy per nucleon
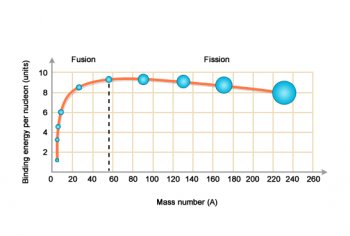
It takes energy, called binding energy, to hold nucleons together as a nucleus.
Iron has a mass number of 56 and is one of the most stable of all the elements. We say that iron has a high binding energy per nucleon.
Elements with lower and higher mass numbers per nucleon are less stable.
The total mass of a nucleus is less than the total mass of the nucleons that make up the nucleus. This difference is known as the mass defect and is equivalent to the binding energy of the nucleus, using E = mc2.
In fission, an unstable nucleus is converted into more stable nuclei with a smaller total mass. This mass defect is the binding energy that is released.
In fusion, the mass of the nucleus that is created is slightly less than the total mass of the original nuclei. Again the mass defect is the binding energy that is released, since the nucleus that is formed is more stable.
Use the following steps in all calculations using E = mc2
Step 1: Check that the mass numbers and atomic numbers are balanced for the reaction.
Step 2: Calculate the total mass before the reaction.
Step 3: Calculate the total mass after the reaction.
Step 4: Calculate the mass defect.
Step 5: Calculate the energy released using E = mc2.
For steps 2 and 3 you will be given the masses of the reactants and the products as needed. Some masses are given in the table.
Calculate the energy released in the following fission reaction.
AnswerStep 1:
The atomic numbers and mass numbers have been checked
Step 2:
Total mass before the reaction = (390.173 + 1.675) × 10-27
= 391.848 × 10-27 kg
Step 3:
Total mass after the reaction= (154.248 + 233.927 + (2 × 1.675)) × 10-27
= 391.525 × 10-27 kg
Step 4:
Mass defect= (391.848 - 391.525) × 10-27 = 0.323 × 10-27 kg
Energy released E = mc2 = 0.323 × 10-27 × (3 × 108)2
= 2.9 × 10-11J
Calculate the energy released in the following fusion reaction.
AnswerTotal mass before the reaction = (5.007 + 3.343) × 10-27 = 8.350 × 10-27 kg
Total mass after the reaction = (6.645 + 1.675) × 10-27 = 8.320 × 10-27 kg
YOU MIGHT ALSO LIKE












