 Through various experiments, Charles Augustin de Coulomb found a way to explain the interactions between charged particles, which in turn helped to explain where the stabilities and instabilities of various particles come from. While the entities that hold atoms together within a molecule can be attributed to bonds, the forces that create these bonds can be explained by Coulomb Forces. Thus, the physical basis behind the bonding of two atoms can be explained.
Through various experiments, Charles Augustin de Coulomb found a way to explain the interactions between charged particles, which in turn helped to explain where the stabilities and instabilities of various particles come from. While the entities that hold atoms together within a molecule can be attributed to bonds, the forces that create these bonds can be explained by Coulomb Forces. Thus, the physical basis behind the bonding of two atoms can be explained.
Introduction
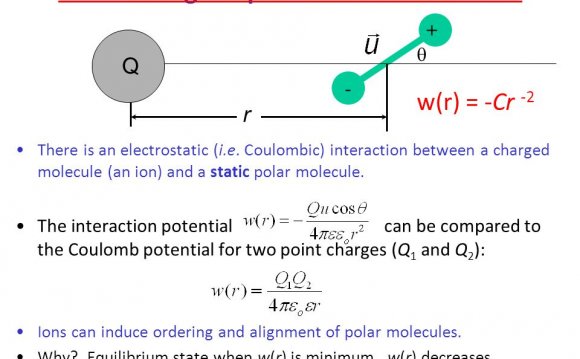
Coulomb’s findings indicate that like charges repel each other and unlike charges attract one another. Thus electrons, which are negatively charged, repel each other but attract protons. Likewise, protons repel each other.
Each atom is made up of a nucleus in the center, which consists of a number of protons and neutrons, depending upon the element in question. Surrounding the nucleus are electrons that float around the nucleus in what can be thought of as a cloud. As two atoms approach one another, the protons of one atom attract the electrons of the other atom. Similarly, the protons of the other atom attract the electrons of the first atom. As a result, the simultaneous attraction of the components from one atom to another create a bond. This interaction can be summarized mathematically and is known as Coulomb’s Law:
\[ F \propto \dfrac{q_{1}q_{2}}{r^{2}}\]
In this mathematical representation of Coulomb's observations, F is the electrical force acting between two atoms with q1 and q2 representing the magnitude of the charges of each atom, and r is the distance between the two atoms. According to the above equation, the electrical force between two atoms is inversely proportional to the square of the distance separating the two atoms.
Coulomb's Law Explained Graphically
As two atoms approach each other, energy is released and thus the overall energy decreases. When the atoms become close enough to the point where repulsive and attractive forces balance each other, energy ceases to be released and as a result the atoms reach the minimum energy level; a stable bond forms. The distance from one nucleus to the other in a stable bond is known as the bond length. When the atoms get too close together, however, the energy begins to rise again due to repulsion between the two nuclei and the two sets of electrons. In order to break a bond, energy must be absorbed. Thus, the energy level of the atoms rise. When the atoms are far apart enough from one another, attractive and repulsive forces are no longer significant and the energy levels of the atoms will approach a constant value.

Problems
- Describe the interactions and processes that occur when a bond is formed.
- What is required to break a bond?
- Translate into words the mathematical representation of Coulomb's Law: \[F \propto \dfrac{q_1q_2}{r^2}\]
- Fill in the blanks: Like charges _____ each other and dislike charges _______ each other.
- What happens when two atoms get too close to one another?
Answers
- The protons in one atom are attracted to the electrons in another atom. The protons in the other atom are attracted to the electrons in the first atom. The two atoms come closer to each other until the the attractive forces between the protons and electrons balance out the repulsive forces between protons and between electrons. As this formation of a bond occurs, energy is released and a stable bond is formed at which the energy level of the bonded atoms is at a minimum.
- Energy must be absorbed in order to break the bond between two atoms.
- Electrical force between two atoms is inversely proportional to the square of the distance separating the two atoms.
- Like charges repel each other and dislike charges attract each other.
- When two atoms get too close to one another, the protons in one atom repel the protons in the other atom and the electrons in one atom repel the electrons in the other atom. The overall energy increases.
References
- Gabler, Raymond. Electrical Interactions in Molecular Biophysics. New York: Academic Press, Inc., 1978.
- Hewitt, Paul G. Conceptual Physics . New York: Harper Collins, 1993.
YOU MIGHT ALSO LIKE












