
Nuclei of the same element having same atomic number but different mass number are known as isotopes.
ORNuclei of same element having same number of proton but different number of neutron are knows as isotopes.
Example
- 17Cl35 , 17Cl37
- 6C12 , 6C13
ISOTOPES OF HYDROGEN
There are three isotopes of hydrogen.
- Protium
- Deuterium
- Tritium
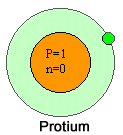
Protium
Ordinary hydrogen is knows as protium .
- It has one electron, one proton but it has no neutron.
- Mass number : 1
- Charge number : 1
- Symbol : 1H1
Percentage in natural hydrogen
99.98%
Structure
 Deuterium
Deuterium
Heavy hydrogen is known as deuterium.
- It has one electron one proton and one neutron.
- Mass number: 2
- Charge number: 1
- Symbol: 1H2 or D
0.0156%
Ratio
Protium:Deturium =1:15000
StructureHeavy water(D2O) consists of deuterium isotope of hydrogen.
TRITIUM
- It has one electron, one proton and two neutrons.
- Mass number : 3
- Symbol : 1H3 or 1T3
4 X 10-15 %
- It is a radioactive isotope.
- It emits B-rays.
- It has a half life of 12.5 years.
- It is present in traces.









