
Characteristics of X-Rays
- X-rays are electrically neutral. They have neither a positive nor a negative charge. They cannot be accelerated or made to change direction by a magnet or electrical field.
- X-rays travel at the speed of light in a vacuum.
- X-rays cannot be optically focused.
- X-rays form a polyenergetic or heterogenous beam.
- The x-ray beam used in diagnostic radiography comprises many photons that have many different energies.
- X-rays travel in straight lines.
- X-rays can cause some substances to fluoresce.
- X-rays cause chemical changes to occur in radiographic and photographic film.
- X-rays can be absorbed or scattered by tissues in the human body.
- X-rays can produce secondary radiation.
- X-rays can cause chemical and biologic damage to living tissue.
X-ray Production
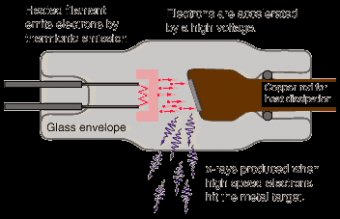
The production of x-rays requires a rapidly moving stream of electrons that are suddenly decelerated or stopped. The source of electrons is the cathode, or negative electrode. Electrons are stopped or decelerated by the anode, or positive electrode. Electrons move between the cathode and the anode because there is a potential difference in charge between the electrodes.Cathode
- Negatively charged electrode
- Consists of a filament and a focusing cup. Filament is a coiled tungsten wire that serves as the source of electrons during x-ray production.
- Most x-ray tubes are referred to as dual-focus tubes because they use two filaments; a large and a small. Only one filament is energized at any one time during x-ray production.
- Focusing cup is made of nickel and mostly surrounds the filament to focus the stream of electrons before they strike the anode.
Anode
- Positively charged electrode
- Consists of a target and in rotating anode tubes, a stator and rotor.
- The target stops the electrons and creates the opportunity for the production of x-rays.
- The target of rotating anode tubes is made of tungsten and rhenium alloy.
- Tungsten generally makes up 90% of the composition of the rotating target, with rhenium making up the other 10%.
- Rotating anodes generally have a target angle ranging from 6 to 20 degrees.\
- Tungsten is used as the material of choice for the rotating targets because of its high atomic number of 74 and a high melting point of 3370 degrees F.
- Anodes rotates from 3, 300 rpm to 10, 000 rpm.
Some additions to this basic set up include the anode hood made of copper and tungsten that act like blinkers to prevent stray electrons from striking the walls of the tube. The copper catches the electrons and the tungsten attenuates the photons produced in the copper. The window is thin and made of beryllium. Beryllium is chosen because it is a metal which has little effect on the photon beam and can effectively maintain the vacuum.
X-Ray Production
X-rays are produced by two main mechanisms and come in two varieties - characteristic and bremsstrahlung xraysCharacteristic xrays
Electrons are the same whether orbiting in shells around the nucleus, or produced inside an xray generator. When ever their velocity or position is changed, there is a loss of energy that takes a radiative form (xrays). When electrons travelling at the target have their direction changed, a spectrum of xrays results. However the electrons circulating in the atoms can also change.
While most of the electrons have their path changed and little else, some will collide with electrons. Sufficient energy in such collisions can result in the ejection of an orbiting electron. 'Sufficient energy' means enough to overcome the bonding energy of the orbiting electron. The impacting electron will move off with reduced energy, and the ejected electron will move off in a different direction and speed with the remaining energy, there is an empty position in one of the shells. The remaining orbiting electrons will 'pack down' to fill the hole, and when changing orbits will lose energy and emit this as radiation. The orbiting levels are fixed as a physical property fixing the elemental identity of an atom, and so the energy emission will be characteristic of that atom. The energy will be mono-energetic and so appear as a spike rather than a continuous spectrum. Electrons ejected come from the K, L or M orbits. The other corollary of this type of interaction is that the atom becomes an ion (it has lost an ejected electron!).
All atoms will produce characteristic radiation but not all are visible in the xray portion of the electromagnetic spectrum. Elements with higher atomic numbers have their K, L, M or N shells of sufficient energy to be called 'xrays'. The discrete characteristic radiation energies are equal to the difference in the energy level of the outer and inner orbital electrons.
The xray energy is proportional to the atom's Z. Where the incident electrons have energies less than the electron binding energy, there will be no characteristic radiation emitted. As the electron energy increases past the threshold level, the maximum level of characteristic radiation reaches 20% of total production, and then starts to fall to 10% in the 50-100 keV range and 3% in the 200 keV range. In the megavoltage range, characteristic radiation is negligible.
Bremsstrahlung xrays
Bremsstrahlung is a German word meaning “braking radiation” which describes the process of xray generation. The high speed electron impacts on the target and at the atomic level approaches the nucleus. There is no actual collision between electron and nucleus because the electron interacts with the Coulombic nuclear forces and its vector quantities of direction and velocity are changed. Since kinetic energy derives from velocity (KE=1/2mv^2). The change in energy is radiated as electromagnetic radiation. The amount of energy means a short wavelength within the xray band.
As the electron is not destroyed, it can undergo multiple interactions, and even initial interactions will vary from minor to major energy changes depending on the actual angle and proximity of attack, and the point of 'impact' on the nucleus. As a result, bremsstrahlung radiation will have continuous spectrum where the maximum energy relates to the entire KE of the electron but will be infrequent. The energy spectrum without filtration is a straight line that matches the formula
whereIE = intensity of photons of energy E
is a constant,
Z is the atomic number of the target,
Em is the maximum photon energy which is numerically equal to the applied kilovolts peak (kVp).The average energy of a bremsstrahlung-derived beam is approximately 1/3 of the maximum energy (or kVp).
The direction of bremsstrahlung xrays is decidedly horticultural. Where the energy (kVp) of the incident electron beam is around 100 keV, bremsstrahlung production has a spatial orientation described as 'anisotropic', that is equally in all directions.
https://www.astash.com website design company Phoenix AZ top web designers in Phoenix.
YOU MIGHT ALSO LIKE












